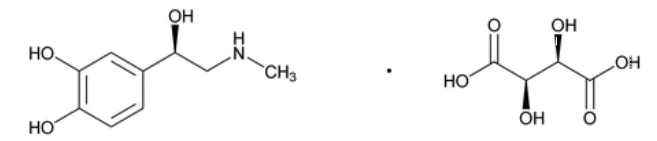欧洲药典(EP)中的英文名为Adrenaline Tartrate
CAS No.: 51-42-3
结构式:
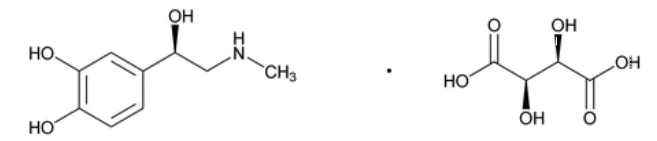
分子式:C9H13NO3 · C4H6O6
分子量:333.29
1,2-Benzenediol, 4-[1-hydroxy-2-(methylamino)ethyl]-, (R)-,
[R-(R*, R*)]-2,3-dihydroxybutanedioate (1:1) (salt);
(-)-3,4-Dihydroxy-α-[(methylamino)methyl] benzyl alcohol
(+)-tartrate (1:1) salt [51-42-3]. UNII: 30Q7KI53AK
Change to read:
DEFINITION
Epinephrine Bitartrate contains NLT 97.0% and NMT ▲103.0%▲ (USP 1-May-2021) of Epinephrine Bitartrate (C9H13NO3·C4H6O6), calculated on the dried basis.
IDENTIFICATION
Delete the following:
▲• PROCEDURE
Sample: 500 mg
Analysis: Dissolve the Sample in 20 mL of water containing 100 mg of sodium bisulfite. Add 6 N ammonium hydroxide until the solution has a distinct odor of ammonia, and allow to stand in a refrigerator for 1 h. Filter the precipitate, wash it with three 2-mL portions of cold water, then with 5 mLof cold alcohol, and finally with 5 mL of cold ether, and dryin vacuum over silica gel for 3 h. To 5 mL of pH 4.0 acid phthalate buffer, add 0.5 mL of a slightly acid solution ofabove obtained Epinephrine (1 in 1000) and 1.0 mL of 0.1 N iodine, and allow to stand for 5 min. Add 2 mL of sodium thiosulfate solution (1 in 40). (See Reagents, Indicators, and Solutions–Buffer Solutions.)
Acceptance criteria: A deep red color isproduced. ▲ (USP 1-May-2021)
Add the following:
▲• A. SPECTROSCOPIC IDENTIFICATION TESTS á197ñ, InfraredSpectroscopy: 197A or 197K▲ (USP 1-May-2021)
Add the following:
▲• B. The retention time of the major peak of the Samplesolution corresponds to that of the Standard solution, asobtained in the Assay. ▲ (USP 1-May-2021)
ASSAY
Change to read:
• PROCEDURE
▲Protect the Standard solution and Sample solution from light.
Buffer: 5.0 g/L of potassium dihydrogen phosphate and 2.6 g/L of sodium octanesulfonate in water. Adjust with phosphoric acid to a pH of 2.8. Dilute with water to volume.
Solution A: Acetonitrile and Buffer (5:95)
Solution B: Acetonitrile and Buffer (45:55)
Mobile phase: See Table 1.
Table 1
Time (min) | Solution A (%) | Solution B (%) |
0 | 95 | 5 |
20 | 50 | 50 |
21 | 50 | 50 |
23 | 95 | 5 |
30 | 95 | 5 |
Standard solution: 0.91 mg/mL of USP Epinephrine Bitartrate RS in Solution A
Sample solution: 0.91 mg/mL of Epinephrine Bitartrate in Solution A
Chromatographic system
(See Chromatography <621>, System Suitability.)
Mode: LC
Detector: UV 210 nm
Column: 4.6-mm × 10-cm; 3-µm packing L1
Temperatures
Autosampler: 4°
Column: 50°
Flow rate: 1.2 mL/min
Injection volume: 5 µL
Run time: NLT 5.6 times the retention time of epinephrine
System suitability
Sample: Standard solution
Suitability requirements
Tailing factor: NMT 2.0
Relative standard deviation: NMT 1.1%
Analysis
Samples: Standard solution and Sample solution
Calculate the percentage of epinephrine bitartrate (C9H13NO3 · C4H6O6) in the portion of Epinephrine Bitartrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of epinephrine from the Sample solution
rS = peak response of epinephrine from the Standard solution
CS = concentration of USP Epinephrine Bitartrate RS in the Standard solution (mg/mL)
CU = concentration of Epinephrine Bitartrate in the Sample solution (mg/mL)
Acceptance criteria: 97.0%–103.0% on the dried basis▲ (USP 1-May-2021)
IMPURITIES
Change to read:
• RESIDUE ON IGNITION á281ñ: ▲NMT 0.1%▲ (USP 1-May-2021)
Delete the following:
▲• LIMIT OF ADRENALONE
Sample: 4 mg/mL of Epinephrine Bitartrate in dilute hydrochloric acid (1 in 200)
Instrument conditions
Mode: UV
Analytical Wavelength: 310nm
Cell: 1cm
Analysis: Determine absorptivity.
Acceptance criteria: NMT 0.2▲ (USP 1-May-2021)
Delete the following:
▲• LIMIT OF NOREPINEPHRINE BITARTRATE
Standard stock solution A: 200 mg/mL of USP Epinephrine Bitartrate RS in water
Standard solution A: 20 mg/mL of USP Epinephrine Bitartrate RS in methanol, from Standard stock solution A
Standard stock solution B: 8.0 mg/mL of USP Norepinephrine Bitartrate RS in water
Standard solution B: 0.80 mg/mL of USP Norepinephrine Bitartrate RS in methanol, from Standard stock solution B
Sample solution: 20 mg/mL of Epinephrine Bitartrate in a mixture of methanol and water (90:10)
Chromatographic system
(See Chromatography á621ñ, Thin-Layer Chromatography.)
Mode: TLC
Adsorbent: 0.25-mm layer of chromatographic silica gel mixture
Application volume: 5 µL
Spray reagent: Folin-Ciocalteu Phenol TS, followed by sodium carbonate solution (1 in 10)
Developing solvent system: n-Butanol, water, and formic acid (70:20:10)
Analysis
Samples: Standard solution A, Standard solution B, and Sample solution
Spray the plate. The RF value of the principal spot of the Sample solution corresponds to that of Standar solution A. Any spot of the Sample solution is not larger nor more intense than the spot with the same RF value of Standard solution B.
Acceptance criteria: NMT 4.0% of norepinephrine▲ (USP 1-May-2021)
Add the following:
▲• ORGANIC IMPURITIES
Protect the Standard solution and Sample solution from light.
Buffer, Solution A, Solution B, and Mobile phase: Prepare as directed in the Assay.
Standard stock solution: 1.5 mg/mL of USP Epinephrine Bitartrate RS in a mixture of 0.1 M hydrochloric acid and Solution A (10:90). [NOTE—If necessary, sequentially add 0.1 M hydrochloric acid to 10% of the flask volume, then dilute with Solution A, to aid in complete dissolution.]
System suitability solution: 15 µg/mL each of USP Norepinephrine Bitartrate RS, USP Adrenalone Hydrochloride RS, and USP Epinephrine Bitartrate RS, prepared as follows. Transfer a suitable amount of USP Norepinephrine Bitartrate RS and USP Adrenalone Hydrochloride RS into a suitable volumetric flask. Add asuitable volume of Standard stock solution into the volumetric flask. Dilute with Solution A to volume.
Standard solution: 1.5 µg/mL of USP Epinephrine Bitartrate RS. Dilute with Solution A from the Standard stock solution.
Sensitivity solution: 0.75 µg/mL of USP Epinephrine
Bitartrate RS in Solution A from the Standard solution
Sample solution: 1.5 mg/mL of Epinephrine Bitartrate in a mixture of 0.1 M hydrochloric acid and Solution A (10:90). [NOTE—If necessary, sequentially add 0.1 M hydrochloric acid to 10% of the flask volume, then dilute with Solution A, to aid in complete dissolution.]
Chromatographic system: Proceed as directed in the Assay, except for the Injection volume.
Injection volume: 20 µL
System suitability
Samples: System suitability solution, Standard solution, and Sensitivity solution
Suitability requirements
Resolution: NLT 3.0 between norepinephrine and epinephrine, System suitability solution Relative standard deviation: NMT 2.0%, Standard solution
Signal-to-noise ratio: NLT 10, Sensitivity solution
Analysis
Samples: Standard solution and Sample solution Calculate the percentage of each specified or any unspecified impurity in the portion of Epinephrine
Bitartrate taken:
Result = (rU/rS) × (CS/CU) × 100
rU = peak response of each specified or any unspecified impurity from the Sample solution
rS = peak response of epinephrine from the Standard solution
CS = concentration of USP Epinephrine Bitartrate RS in the Standard solution (mg/mL)
CU = concentration of Epinephrine Bitartrate in the Sample solution (mg/mL)
Acceptance criteria: See Table 2. The reporting threshold is 0.05%.
Table 2
Relative Retention Time | Acceptance Criteria, NMT (%) | |
Norepinephrine a | 0.8 | 0.15 |
Epinephrine | 1.0 | - |
Adrenalone b | 1.3 | 0.2 |
Unidentified impurity peak 1 c | 3.2 | 0.3 |
Any unspecified impurity | - | 0.10 |
Total impurities | - | 0.6 |
a (R)-4-(2-Amino-1-hydroxyethyl) benzene-1,2-diol.
b 3′,4′-Dihydroxy-2-(methylamino)acetophenone.
c Unknown structure, and identified based on relative retention time.▲ (USP 1-May-2021)
Add the following:
▲• ENANTIOMERIC PURITY
Protect the Standard solution and Sample solution from light.
Buffer: Dissolve 1.2 g of monobasic sodium phosphate in900 mL of water and adjust with dilute sodium hydroxide to a pH of 5.8. Dilute with water to 1000 mL. Add 18.6 mg of EDTA disodium salt dihydrate and mix.
Mobile phase: Isopropyl alcohol and Buffer (5:95)
Standard stock solution A: 1.8 mg/mL of USP Epinephrine Bitartrate RS in 1 N hydrochloric acid and Mobile phase (2:98)
Standard stock solution B: 0.96 mg/mL of USP Racepinephrine Hydrochloride RS in Mobile phase
System suitability solution: 1.8 mg/mL of USP Epinephrine Bitartrate RS and 0.072 mg/mL of USP Racepinephrine Hydrochloride RS prepared as follows. Transfer a suitableamount of USP Epinephrine Bitartrate RS into a suitable volumetric flask. Add Standard stock solution B to 7.5% ofthe flask volume and 1 N hydrochloric acid to 2% of the flask volume. Dilute with Mobile phase to volume.
Standard solution: 0.054 mg/mL of USP Epinephrine Bitartrate RS in Mobile phase from Standard stock solution A
Sample solution: 1.8 mg/mL of Epinephrine Bitartrate in a mixture of 1 N hydrochloric acid and Mobile phase (2:98)
Chromatographic system
(See Chromatography á621ñ, System Suitability.)
Mode: LC
Detector: UV 280 nm
Column: 4.0-mm × 10-cm; 5-µm packing L108
Autosampler temperature: 4°
Flow rate: 0.9 mL/min
Injection volume: 10 µL
Run time: NLT 10 times the retention time of S-epinephrine
System suitability
Samples: System suitability solution and Standard solution
[NOTE—The relative retention times of R-epinephrine and S-epinephrine are about 1.0 and 1.25, respectively.]
Suitability requirements
Resolution: NLT 1.5 between R-epinephrine and S-epinephrine, System suitability solution Relative standard deviation: NMT 2.0% for R-epinephrine, Standard solution
Analysis
Sample: Sample solution
Calculate the percentage of S-epinephrine bitartrate in the portion of Epinephrine Bitartrate taken:
Result = (rU/rT) × 100
rU = peak response of S-epinephrine
rT = sum of the peak responses of R-epinephrine and S-epinephrine
Acceptance criteria: NMT 3.0%▲ (USP 1-May-2021)
SPECIFIC TESTS
Delete the following:
▲
• MELTING RANGE OR TEMPERATURE á741ñ: 147°–152°, with decomposition▲ (USP 1-May-2021)
Change to read:
• LOSS ON DRYING <731>
▲Sample: 1 g
Analysis: Dry under vacuum for 18 h or until constant weight.
Acceptance criteria: NMT 0.5%▲ (USP 1-May-2021)
Delete the following:
▲• OPTICAL ROTATION, Specific Rotation <781>: −50.0° to
−53.5°
Sample solution: 20 mg/mL of above obtained epinephrine, in hydrochloric acid solution (1 in 20)▲ (USP 1-May-2021)
ADDITIONAL REQUIREMENTS
• PACKAGING AND STORAGE: Preserve in tight, light-resistant containers.
Change to read:
• USP REFERENCE STANDARDS <11>
▲USP Adrenalone Hydrochloride RS
3′,4′-Dihydroxy-2-(methylamino) acetophenone hydrochloride.
C9H11NO3 · HCl 217.65▲ (USP 1-May-2021)
USP Epinephrine Bitartrate RS
USP Norepinephrine Bitartrate RS
▲USP Racepinephrine Hydrochloride RS (RS)-4-(1-Hydroxy-2 (methylamino)ethyl) benzene-1,2- diol hydrochloride.
C9H13NO3 · HCl 219.67▲ (USP 1-May-2021)